CDMO
- Home
- CDMO
Contract Development and Manufacturing Organization
Expansion Into Contract Manufacturing: Our Next Chapter
In our commitment to innovation and growth, Agora Alliance SL, in collaboration with Andhra Medi Pharma India Private Limited, has founded Biofern Life Sciences, a contract development and manufacturing organisation (CDMO) based in Bengaluru, India. Specialising in nutraceuticals, herbaceuticals, and organic products, Biofern Life Sciences offers tailor-made solutions for vitamins, minerals, health supplements, and specialty tablets.
With a relentless pursuit of innovation and quality, we proudly stand as India’s pioneering manufacturer of custom-made healthcare solutions for global markets. Through Biofern, we expand our capabilities to provide contract manufacturing services, further empowering your business endeavours.
At Biofern Life Sciences, we are committed to revolutionising the contract manufacturing landscape.

Research & Development (R&D) and New Product Development
At Biofern, we pride ourselves on our collaborative approach to R&D and new product development, working closely with our customers to bring their visions to life and create successful products tailored to their needs.
Our Process:
- Concept Formulations: Our Product Development Manager utilises trend and market analysis, coupled with new supplier assessments, to provide concept formulations for both new product development and existing product optimization.
- Refinement and Development: These initial formulations undergo further refinement and development under the expertise of our in-house product development team. Piloted and production-trialled in our fully equipped small-scale pilot facility and extensive development laboratories, we ensure precision and efficacy at every stage.
- Scale-Up Manufacturing: Leveraging our extensive internal expertise and experience within our production team, we seamlessly transfer the product to full-scale production. Throughout this process, we finalise product, process, and packaging specifications to meet the highest standards of quality and compliance.








Production & Facilities:
Our state-of-the-art facilities are equipped with advanced technology to ensure efficient and precise manufacturing processes. With humidity-controlled dispensing bays, wet and dry granulation, high-speed compression machines, and film and sugar coating capabilities, we guarantee optimal product quality and consistency. Our isolation suite further ensures the segregation of ingredients for enhanced safety and purity.
Manufacturing Capacity (Daily):
– Granulation: 250 kgs – 3MT, 120 kgs – 2.4MT
– Tablets: 2×51 stn high-speed compression d-tooling – 10 million
– Coating: 60” – 3.7 million, 48” – 2.1 million
– Capsules: PF90 – 4 million
– Blister: 4.3 million tablets
– Bottles: 2.5 million
– Powders: 200gms – 58,000 jars, 500gms – 45,000 jars, 1kg – 24,000 jars, 2kgs – 15,000 jars
– Sachets: 0.216 million
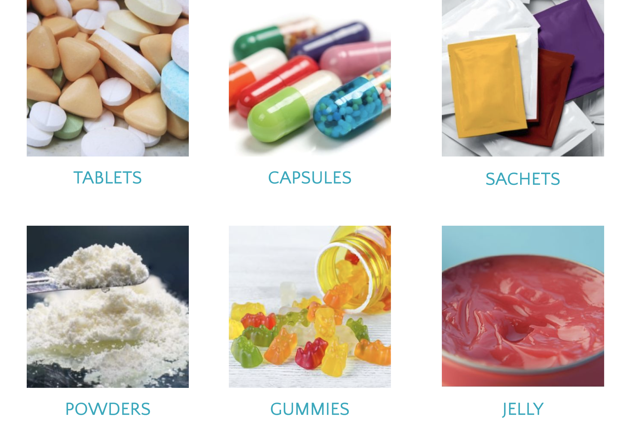
Dosage Formats:
At Biofern Life Sciences, we offer a diverse range of dosage formats tailored to meet your specific requirements:
– Tablets: Available in bespoke or standard formulations
– Capsules: Offered with powder and beadlet fills in various sizes
– Sachets: Ranging from 1g to 40g for convenient usage
– Powders: Blending facility for an extensive range of products
– Gummies & Jelly: Coming soon to expand our product offerings





Quality Assurance & Control:
Our commitment to quality is unwavering. End-to-end testing of raw materials, in-process, and finished products is conducted by our highly trained quality team at every stage of production. Adhering to G.M.P. and ISO protocols, we ensure zero contamination and uphold the highest standards of product integrity. With well-qualified professionals and decades of experience in quality management, cGMP compliance, audit management, and validation management, our quality assurance and control teams guarantee the delivery of premium-quality products to the market.


